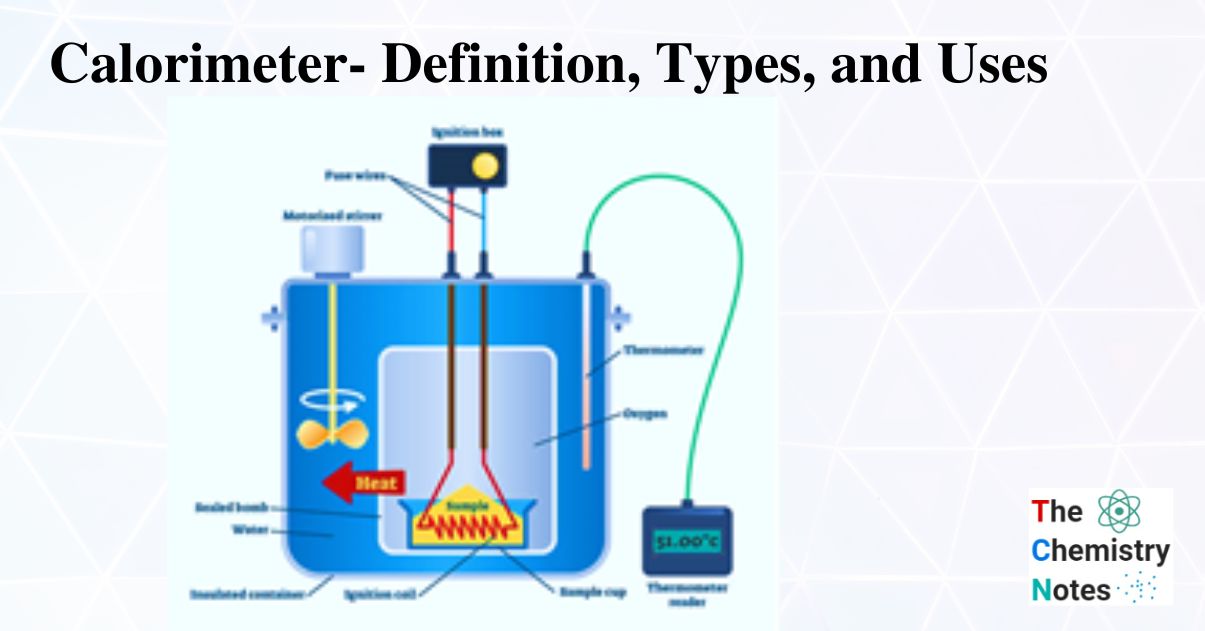
Why Is Calorimetry Important . One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Apply the first law of thermodynamics to calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in.
from thechemistrynotes.com
One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. By knowing the change in.
Calorimeter Definition, Types and Uses
Why Is Calorimetry Important Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. Apply the first law of thermodynamics to calorimetry. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. By knowing the change in. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. A calorimeter is a device used to measure the amount of heat involved in a. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important For example, when an exothermic. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the process of measuring the. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing the change in. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern.. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. For example, when an exothermic. Compare. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic.. Why Is Calorimetry Important.
From bettyelevisxo.blob.core.windows.net
Fundamentals Of Calorimetry Lab Why Is Calorimetry Important One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry literally means “heat measurement.” for consistency with other forms of. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important For example, when an exothermic. Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. By knowing the change in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus. Why Is Calorimetry Important.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Why Is Calorimetry Important For example, when an exothermic. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in. Why Is Calorimetry Important.
From saylordotorg.github.io
Calorimetry Why Is Calorimetry Important By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process. Why Is Calorimetry Important.
From studylib.net
Calorimetry Why Is Calorimetry Important Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can. Why Is Calorimetry Important.
From helgroup.com
The Fundamentals of Calorimetry H.E.L Group Why Is Calorimetry Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. By knowing the change in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Why Is Calorimetry Important.
From study.com
Calorimetry Definition, Equation & Types Lesson Why Is Calorimetry Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Calorimetry. Why Is Calorimetry Important.
From www.studocu.com
Experiment 6 Calorimetry Experiment 6 Calorimetry In this experiment Why Is Calorimetry Important Calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the modern. For example, when an exothermic. By knowing the change in. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a field of thermochemistry that measures the amount. Why Is Calorimetry Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to. Why Is Calorimetry Important.